CIP / SIP
Sectors
- Pharmaceutical
- Biopharma
- Food Industry
- Cosmetics
- Other Aseptic Process Industries
Talk to us
For more information about our solutions why not download our brochures
CIP & SIP systems are designed and built to clean process equipment, transfer lines, formulation reactors and many other systems, in full compliance with cGMP guidelines in accordance with FDA and EP requirements.
Engineering and manufacturing practices follow ISO 9001 procedures and comply with internationally recognised standards and guidelines, such as ASME BPE and GAMP. The design and build adhere to regulations and guidelines from the EU and U.S.
Puretech works in partnership with its clients to ensure every unit is designed for their needs and specific application to provide repeatable and validatable CIP cleaning. As standard included are DQ, IQ and OQ documentation and Factory Acceptance Testing
Features & Benefits
- Skid mounted units designed to minimise footprint
- Fully automated operation and easily accessibly connections
- Fully automated control system
- Fully programmable multiple cleaning cycles
- Systems come complete with FAT and IQ/OQ documentation as standard and execution is available on request.




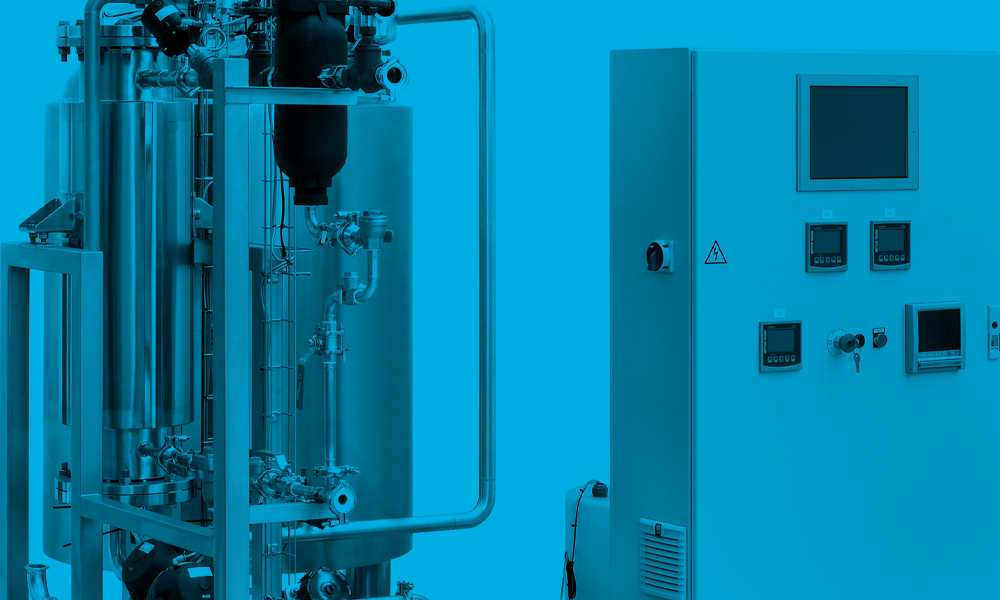
Key Components
Storage tanks
- 316L stainless steel vessels are used as a reservoir for CIP cycles. Cleaning solutions are prepared utilising the vessel before the solution is sent to the equipment.
- Insulated walls reduce utilities and heat loss.
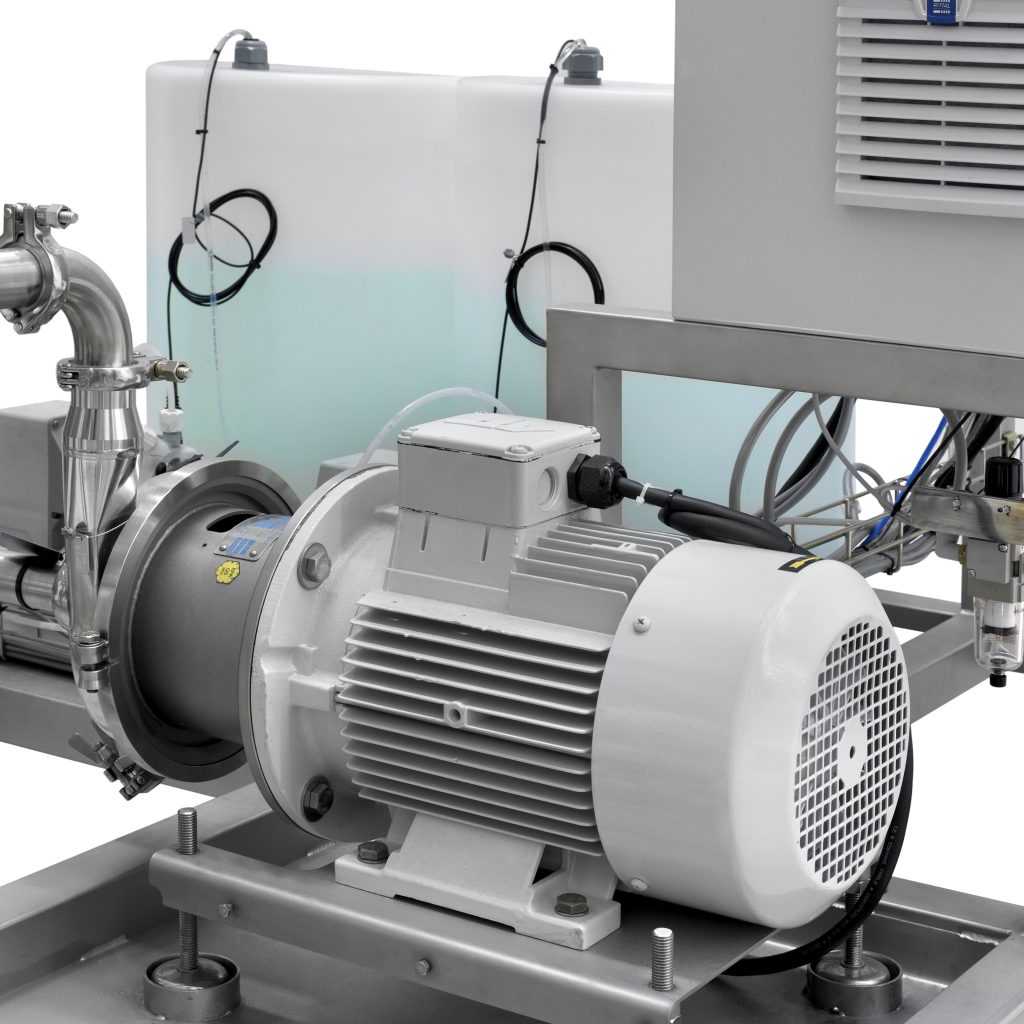
Centrifugal pumps
- 316L SS sanitary centrifugal pump with inverter, ensures CIP parameters are achieved as well as minimising energy costs and reducing cleaning times.
- Flexible outputs from 500 l/h to 15,000 l/hrn between 1 bar to 7 bar (other flows and pressure are available on request)
Cooling/Heat exchanger
- Steam heated/cooling water units, double plate or shell and tube sanitary heat exchangers available to achieve required temperatures.
- Electrical heating available on request
Chemical Dosing
- Dosing systems can be added to the system as an acid, alkali, anti-foaming or special cleaning agents. Concentration can be modified depending on the recipe.
Instrumentation
- Critical parameters are controlled by various sensors &transmitters. to insure an effective cleaning procedure with the correct pH, conductivity & Temperature.
- Provides constant monitoring of Flow, Temperature, Pressure, pH, Conductivity, and TOC (option)
Operating principle
Pre-configured cleaning processes
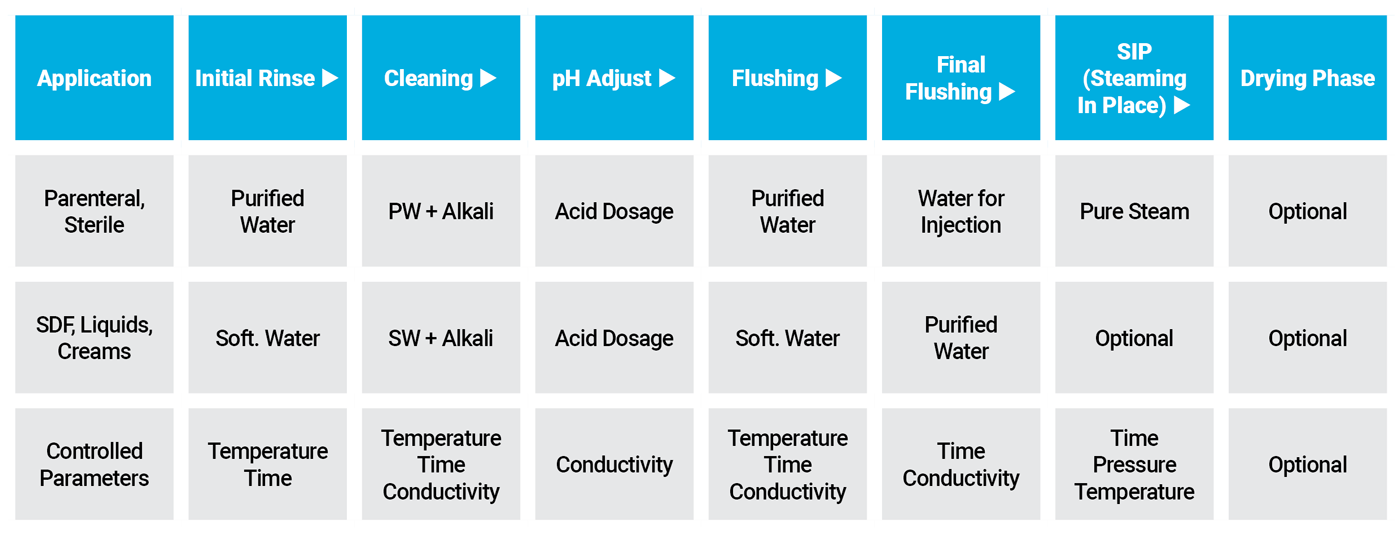
Cleaning Procedure and Recipes are defined together with the client during the design phase
Cleaning procedure must be approved and validated.
- Initial Rinse to eliminate residues
- Alkaline cleaning procedure: Alkaline detergents dissolve fat and/or proteins
- pH adjustment to neutralise the caustic left on surfaces of the equipment. Acid dosing removes mineral deposits
- Flushing: Purified Water or Water for injection is used to flush the residual acid solution away.
- Steam in place (if required)
Control System
- An HMI allows ease of operation and monitoring with the following parameters
- Mimics of the equipment, showing the operational status in real time
- Process Parameters (temperature, pressure, Conductivity, pH)
- System configuration
- Alarm notification & Acknowledgment
- Control and monitoring with data logging facilities and connections to central systems such as BMS, DCS, and SCADA
- GAMP and 21 CFR part 11 compliant
- Recipe management
Mobile Units
Design & construction features
Features & Benefits
- Compact Units designed for easy transportation and storage within production areas
- Fully automated operation and easily accessibly connections
- Fully programmable multiple cleaning cycles
- Systems come complete with FAT and IQ/OQ documentation as standard and execution is available on request.
Service & Maintenance
Puretech have nearly 30 years’ experience of servicing and maintaining pharmaceutical, validated water systems.
Our service contracts are tailored to our clients individual needs and the service is expertly provided through our network of experienced and qualified engineers.
We can offer contracts of 1-5 years with the numbers of visits specified by you. All of our contracts as standard receive 24/7 telephone support, recommended spare parts list and a detailed service report to ensure you comply with all regulatory body standards.
Let’s Discuss How We Can Work Together!
We would welcome the opportunity to discuss how Puretech can become your trusted partner. We’d love to schedule a meeting to explore potential synergies and discuss how we can support your design projects with our expertise.
Please contact us to schedule a meeting:
- Email: sales@puretech.uk.com
- Phone: +44 (0) 1737 378 000
- Website: www.puretech.uk.com






